Abstract
Background: Venetoclax (VEN)-based combination therapy with hypomethylating agents (HMA) has been approved for first-line treatment in AML patients ineligible for intensive treatment. There is evidence for efficacy also in the in relapsed/ refractory setting (R/R) in fit patients pretreated with intensive regimes, but comparative controlled data is lacking.
Here, we report our updated data of VEN-based salvage treatment as bridge to allogeneic hematopoietic cell transplantation (allo-HCT) in R/R AML patients and compared it to non-VEN-based intensive treatment according to physician's choice in a propensity score matching analysis using patient data from the German Study Alliance Leukemia (SAL) registry (ClinicalTrials.gov Identifier: NCT03188874).
Design/Methods: We analyzed patients with R/R AML after initial intensive induction therapy, who started VEN-based salvage treatment as bridging to allo-HCT at the University Hospital Heidelberg between October 2018 and April 2021. Using propensity score matching, these patients were compared to patients from the registry of the SAL, who received non-VEN-containing salvage treatment based to the decision of the treating physician.
Results: A total of 37 patients (median age 62 y, range 23 to 79 y), who received VEN-based salvage treatment were included. All patients had initially received intensive therapy, 25 patients (68 %) had been refractory to an intensive induction therapy regime, 12 patients (32 %) had been diagnosed with morphologic (9 patients) or molecular relapse (3 patients) after intensive first line therapy. According to the 2017 ELN classification, 10 patients (27%) had favorable risk, 10 (27%) intermediate risk and 17 (46%) adverse risk disease. 36 patients received VEN in combination with standard-dose azacytidine and one patient with low-dose cytarabine. VEN was given at a daily dose of 400mg/day in 21 to 28 days cycles after initial ramp up or at a reduced dose of 100mg/day in case of co-medication with strong CYP3A inhibitors.
The overall response rate (CR, CRi, MLFS and PR) in VEN-based treated patients was 89% (n=33) with CR/CRi being the best response in 57% (n=21), MLFS in 19% (n=7) and PR in 13% (n=5) of the patients. 30- and 60-day mortality was 3% (n=1).
Eventually, allo-HCT was performed in 26 patients (70%). At the time of analysis 17 (65%) of these patients were alive and 16 (62%) were still in CR.
For comparison, we identified 88 patients (median age 61 y; range 22- 78 y) within the SAL-registry, who received an intensive non VEN-containing salvage therapy for their R/R AML. The SAL cohort contained more patients with intermediate risk (ELN 2017 classification) than the VEN-treated cohort (56% vs. 27%; p=0.005) but less patients with adverse risk (22% vs. 46%; p=0.009). The distribution of favorable risk was similar (23% vs. 27%; p=0.65). Allo-HCT was performed in 59 patients (67%) and in 31 patients allo-HCT was the first salvage treatment after relapse (n=10) or refractoriness to induction therapy (n=21). The remission status prior to allo-HCT was CR/CRi in 11 (19%), PR in 7 (12%), progressive disease (PD) in 38 (64%) and unknown in 3 patients (5%).
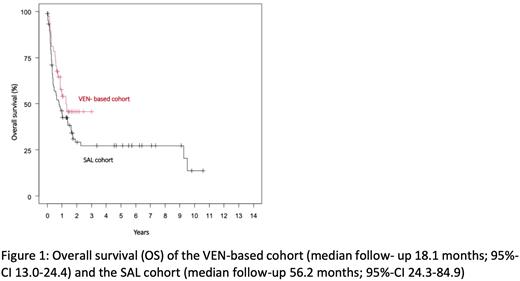
After a median follow-up of 18.1 months (95%-confidence interval (CI) 13.0-24.4) for the VEN-treated cohort and 56.2 months (95%-CI 24.3-84.9) for the SAL cohort, the median event-free survival (EFS) and relapse-free survival (RFS) of VEN-treated patients were 8.02 months (95%-CI, 5.06-not reached [NR]) and 7.16 months (95%-CI, 4.37-NR), respectively. In contrast, median EFS and RFS for the SAL cohort were 4.53 months (95%-CI, 3.45-8.51) and 9.66 months (95%-CI, 5.59-20.6), respectively. Median overall-survival (OS) in the VEN-cohort was 15.08 months (95%-CI, 10.55-NR) compared to 9.66 months (95%-CI, 4.99-19.5) in the SAL cohort. Propensity score matching including age, white cell count, sex and ELN-risk and allo-HCT status as matching variables revealed a non-significant (p=0.148) benefit for the VEN-treated cohort with a hazard ratio of 0.65 (95%-CI, 0.36-1.17) compared to the SAL cohort. In patients proceeding to allo-HCT, 18-month survival was 60% (95%-CI, 42-85%) compared to 51% (95%-CI, 40-66%) in the SAL cohort.
Conclusion: Our data confirms that VEN-containing therapy is a safe and effective salvage therapy for patients with R/R AML that allows an efficient bridging to allo-HCT in many patients with promising outcome after allo-HCT.
Disclosures
Unglaub:Jazz Pharmaceuticals: Other: travel costs. Schlenk:Abbvie: Research Funding; BergenBio: Honoraria; Pfizer: Honoraria, Research Funding; AstraZeneca: Research Funding; Daiichi Sankyo: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; PharmaMar: Research Funding; Novartis: Honoraria. Middeke:Abbvie: Membership on an entity's Board of Directors or advisory committees. Steffen:Jazz Pharmaceuticals: Other: Travel/Congress Participation Support; AbbVie: Other: Travel/Congress Participation Support. Krause:Art-tempi: Honoraria; Kosmas: Honoraria; Abbvie: Other: Expenses. Schliemann:Philogen S.p.A.: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Other: travel grants; Astellas: Consultancy; Astrazeneca: Consultancy; Boehringer-Ingelheim: Research Funding; BMS: Consultancy, Other: travel grants; Jazz: Consultancy, Research Funding; Novartis: Consultancy; Roche: Consultancy; Pfizer: Consultancy. Haenel:Roche: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; GSK: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Bayer: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Pfizer: Honoraria; JAZZ: Consultancy, Honoraria. Jost:Jazz: Honoraria; BMS Celgene: Honoraria. Crysandt:Astra Zeneca: Honoraria; Novartis: Honoraria; Pfizer: Honoraria. Fransecky:Pfizer: Consultancy, Speakers Bureau; Gilead: Consultancy, Research Funding, Speakers Bureau; Abbvie: Consultancy, Research Funding, Speakers Bureau. Einsele:BMS/Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel Grants, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Grants, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel grants; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel grants; Sanofi: Consultancy, Honoraria, Research Funding; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Other: travel grants. Schmid:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kite: Research Funding; Abbvie: Research Funding. Röllig:Celgene Corporation: Consultancy, Honoraria; Jazz Pharmaceuticals: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding; Pfizer Inc.: Consultancy, Honoraria, Research Funding; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria; Servier: Consultancy; Bristol Myers Squibb: Consultancy, Honoraria; Amgen Inc.: Honoraria; AbbVie Inc.: Consultancy, Honoraria, Research Funding. Luft:JAZZ Pharmaceuticals: Honoraria. Dreger:Novartis: Honoraria; Kite: Honoraria. Sauer:Jazz Pharmaceuticals: Honoraria; Abbvie: Honoraria; Pfizer: Honoraria; Ridgeline Discoveries: Consultancy; Gilead: Consultancy; Bristol-Myers Squibb: Consultancy; Amgen: Consultancy; Astellas: Consultancy; Abbvie: Consultancy; Takeda: Consultancy.
OffLabel Disclosure:
Venetoclax as a salvage therapy for relapsed or refractory AML patients, pretreated with intensive regimes
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal